New Jersey, US based Ascend Laboratories has initiated a recall of Metformin Hydrochloride Tablets manufactured by Mumbai based Alkem Laboratories. The product was distributed in the US by Ascend Laboratories.
The Voluntary: Firm Initiated recall is for 1,739 bottles of USP 500 mg, 1000 tablets and has been classified as a Class II Recall – a situation in which use of or exposure to a violative product may cause temporary or medically reversible adverse health consequences or where the probability of serious adverse health consequences is remote.
The recall has been initiated in the 5 states of US alone i.e. Alamaba, Florida, Gerogia, South Carolina and Tennessee.
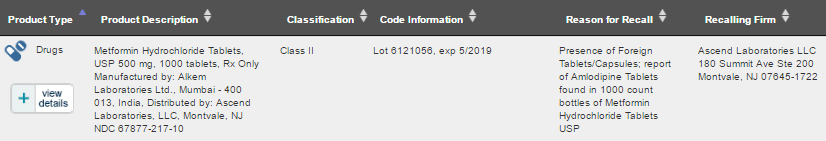
The reason for recall mentioned by Ascend Laboratories was due to the presence of foreign Tablets/Capsules i.e. Amlodipine Tablets used in the treatment of high blood pressure (hypertension) were present in the bottles of Metformin Hydrochloride Tablets.
The batch recalled was from Lot 6121056 with an expiry date of 5/2019.
Alkem Laboratories Recalls 1,739,000 Tablets Of Used For The Treatment Of Oral Diabetes
Like our content? Join Capitalmind Premium.
- Equity, fixed income, macro and personal finance research
- Model equity and fixed-income portfolios
- Exclusive apps, tutorials, and member community
Subscribe Now
Or start with a free-trial
Already a subscriber?
Login Now